Nailfold capillaroscopy in patients with systemic sclerosis-associated interstitial lung disease: A substudy of the SENSCIS trial
RMD Open 2025;11:e005704 Doi:10.1136/rmdopen-2025-005704
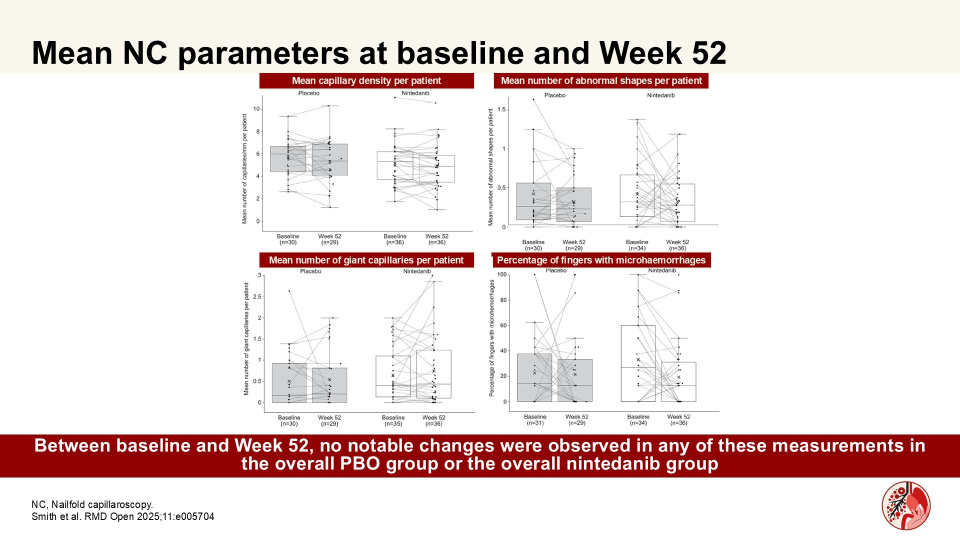
In a substudy of the SENSCIS trial, Smith et al. showed that numerical differences in changes in capillary density assessed by nailfold capillaroscopy (NC) over 52 weeks may suggest a potential effect of nintedanib in patients at risk of ILD progression. Authors assessed microvascular changes in nailfold capillaries in patients with SSc-ILD who received nintedanib or PBO in a sub-study of the SENSCIS trial.
There were no notable changes in capillary density among patients who did not have risk factors for rapid FVC decline at baseline or ILD progression at Week 52. Further research is investigating the utility of NC as a tool for predicting and assessing disease progression in patients with SSc.
Keywords: